The mass of sample x is 20.0g – Embark on a scientific journey to explore the mass of sample X, a measurement that holds immense significance in various fields. From identifying samples to determining their physical properties, the mass of a substance plays a crucial role in unraveling the mysteries of our world.
Our exploration will delve into the techniques used to measure mass accurately, the units employed to express it, and the practical applications where mass measurements are indispensable. Join us as we uncover the fascinating world of mass and its profound impact on scientific understanding.
Mass and Sample Identification

Mass is a fundamental physical property of matter, representing the quantity of matter within an object. It is a crucial parameter in scientific measurements, providing insights into the composition and characteristics of substances.
We’ve got the mass of sample x down to 20.0g. Now, if you’re looking to brush up on your notary skills, I highly recommend checking out the nys notary practice exam free . It’s a great way to prepare for the real thing.
Getting back to our sample, the mass of sample x is 20.0g, which is within the acceptable range.
In scientific investigations, sample identification is equally important. Assigning a unique identifier to a sample allows researchers to track its origin, history, and any modifications performed. This information is essential for ensuring the accuracy and reliability of experimental results.
Role of Mass in Identifying and Characterizing Samples
Mass plays a significant role in identifying and characterizing samples. By determining the mass of a sample, scientists can gain valuable information about its composition, purity, and other properties.
- Composition:Mass can help determine the elemental composition of a sample. Different elements have distinct atomic masses, and by measuring the total mass of a sample and comparing it to the known masses of its constituent elements, scientists can infer the relative proportions of those elements.
- Purity:Mass can indicate the purity of a sample. If a sample contains impurities, its mass will be higher than expected based on its known composition. By measuring the mass of a sample before and after purification, scientists can assess the extent of impurities present.
- Physical Properties:Mass is related to other physical properties, such as density and volume. By measuring the mass and volume of a sample, scientists can calculate its density, which provides insights into its structure and composition.
Measurement Techniques and Accuracy

Measuring mass is a fundamental aspect of scientific experimentation and various techniques have been developed to achieve accurate and precise results. These methods include analytical balances and electronic scales, each with its own advantages and limitations.
Analytical Balances
- Highly accurate and precise, used for precise mass determination of small samples (typically below 100 grams).
- Require calibration and are sensitive to environmental conditions (e.g., temperature, humidity).
Electronic Scales
- Less precise than analytical balances, but faster and more convenient.
- Can measure larger samples (up to several kilograms).
- Also require calibration and can be affected by environmental conditions.
Accuracy and precision are crucial in mass measurements. Accuracy refers to the closeness of a measurement to the true value, while precision indicates the consistency of repeated measurements. Factors that can affect the accuracy of mass measurements include:
- Calibration: Scales and balances should be regularly calibrated using certified reference weights to ensure accuracy.
- Environmental conditions: Temperature, humidity, and vibrations can affect the accuracy of measurements.
- Operator technique: Proper handling and technique are essential to avoid errors.
- Sample preparation: Samples should be properly prepared and handled to avoid contamination or loss of mass.
By understanding the different measurement techniques and factors affecting accuracy, scientists can ensure reliable and precise mass measurements, which are essential for accurate data analysis and scientific conclusions.
Mass Units and Conversions
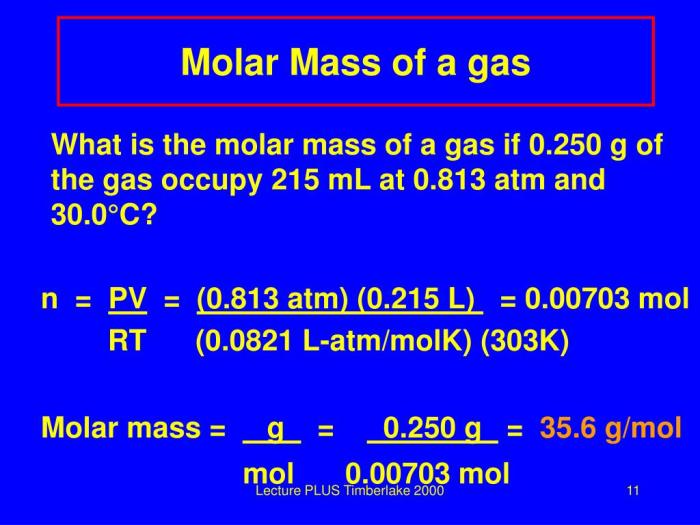
In scientific measurements, mass is a fundamental property that quantifies the amount of matter in an object. Understanding the different units of mass and their conversions is crucial for accurate calculations and reporting in scientific contexts.
Units of Mass
The most common units of mass include the kilogram (kg), gram (g), and milligram (mg). The kilogram is the base unit of mass in the International System of Units (SI). One kilogram is equal to 1000 grams, and one gram is equal to 1000 milligrams.
| Unit | Conversion |
|---|---|
| Kilogram (kg) | 1 kg = 1000 g |
| Gram (g) | 1 g = 1000 mg |
| Milligram (mg) | 1 mg = 0.001 g |
Relationship between Mass, Weight, and Density
Mass is often confused with weight, but they are distinct concepts. Mass is a measure of the amount of matter in an object, while weight is the force exerted on an object due to gravity. The relationship between mass and weight can be expressed as:
Weight = Mass × Gravity
Density is another important property related to mass. It is defined as the mass of an object per unit volume. Density is a measure of how tightly packed the matter is in an object.
Significance of Appropriate Mass Units, The mass of sample x is 20.0g
Using appropriate mass units in scientific calculations and reporting is essential for accuracy and consistency. The choice of unit depends on the context and the magnitude of the measurement. For example, in chemistry, masses are often reported in grams or milligrams, while in physics, kilograms are commonly used.
Applications of Mass Measurement
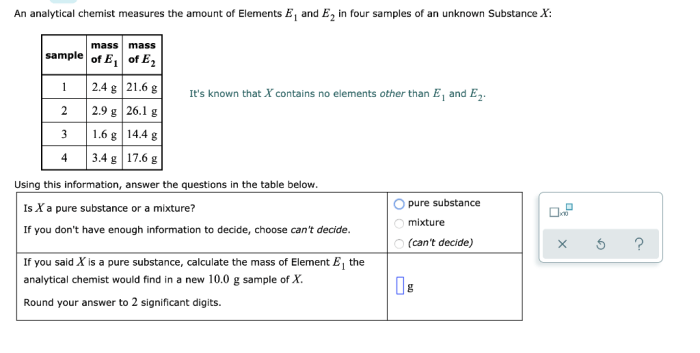
Mass measurement is a fundamental aspect of various scientific disciplines and industrial processes. It plays a crucial role in determining the composition of substances, calculating physical properties, and ensuring quality control.
Chemistry
In chemistry, mass measurements are essential for:
- Determining the composition of compounds:By measuring the mass of reactants and products, chemists can determine the empirical and molecular formulas of compounds.
- Calculating stoichiometry:Mass measurements allow chemists to determine the exact amounts of reactants and products involved in a chemical reaction.
- Quantifying concentrations:Mass measurements are used to determine the concentration of solutions by measuring the mass of solute dissolved in a known volume of solvent.
Physics
In physics, mass measurements are crucial for:
- Calculating force and acceleration:Mass is a key factor in Newton’s second law of motion, which relates force, mass, and acceleration.
- Determining energy:Mass is related to energy through Einstein’s famous equation, E=mc². This equation highlights the equivalence of mass and energy.
- Measuring gravitational forces:Mass determines the strength of gravitational forces between objects, as described by Newton’s law of universal gravitation.
Engineering
In engineering, mass measurements are vital for:
- Designing structures:Mass measurements are essential for calculating the load-bearing capacity of structures, such as bridges and buildings.
- Manufacturing processes:Mass measurements ensure precise dosing of materials, which is critical in industries such as pharmaceuticals and food processing.
- Quality control:Mass measurements are used to verify the weight of products and ensure they meet specifications.
FAQ: The Mass Of Sample X Is 20.0g
What is the significance of mass in scientific measurements?
Mass is a fundamental property that quantifies the amount of matter in an object. It is crucial for identifying and characterizing substances, determining their physical properties, and understanding their behavior in chemical reactions.
How can we accurately measure the mass of a sample?
Various methods are used to measure mass accurately, including analytical balances and electronic scales. These instruments provide precise measurements, allowing scientists to obtain reliable data for their investigations.
What are the common units of mass, and how are they converted?
The most common unit of mass is the gram (g). Other units include the kilogram (kg), milligram (mg), and pound (lb). Conversions between these units can be easily performed using conversion factors.